HECRIN
 HECRIN (Hungarian European Clinical Research Infrastructure Network) aims at supporting the conduct of investigator-initiated multinational clinical trials in Hungary as the national node of ECRIN-ERIC (European Clinical Research Infrastructure Network, www.ecrin.org, European Research Infrastructure Consortium).
HECRIN (Hungarian European Clinical Research Infrastructure Network) aims at supporting the conduct of investigator-initiated multinational clinical trials in Hungary as the national node of ECRIN-ERIC (European Clinical Research Infrastructure Network, www.ecrin.org, European Research Infrastructure Consortium).
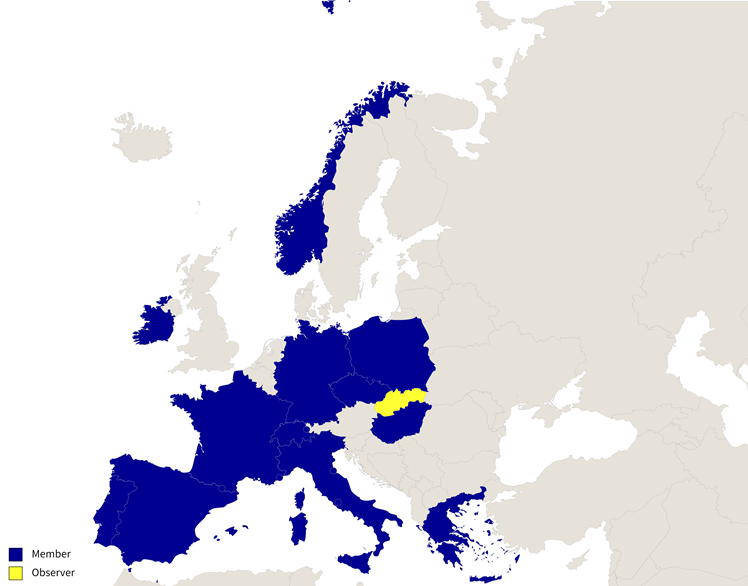
ECRIN-ERIC was created in 2013. Currently it has 12 Member countries (Czech Republic, France, Germany, Greece, Hungary, Ireland, Italy, Norway, Poland, Portugal, Spain, Switzerland) and 1 Observer country (Slovakia). The portfolio of ECRIN-supported trials includes 70+ trials (35 currently active), across all medical specialties, with a mean number of 6 countries per trial.
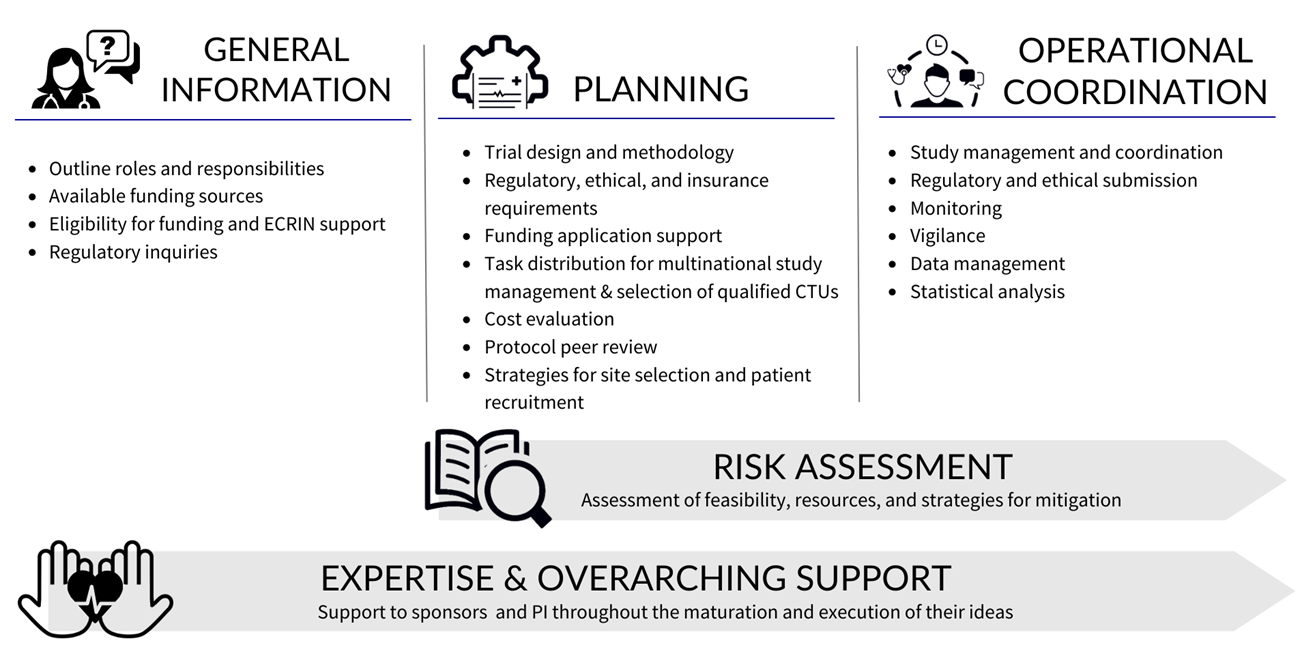
ECRIN supports multinational clinical research through two main mechanisms. First, ECRIN provides services to individual multinational clinical research projects (such as recommendations on trial design and endpoint selection, country and site selection, cost evaluation, ethical, regulatory, data protection and insurance requirements). Once funding is secured, ECRIN can provide sponsor-delegated tasks (ethical and regulatory submissions and follow-up, project management, vigilance, monitoring, data management) through activation of partner Clinical Trial Units (CTUs) in the countries involved and the coordination of their services. Moreover, ECRIN also takes part in development of tools, methodologies and partnerships that help better conduct of academic clinical trials.
ECRIN services connected to clinical trials
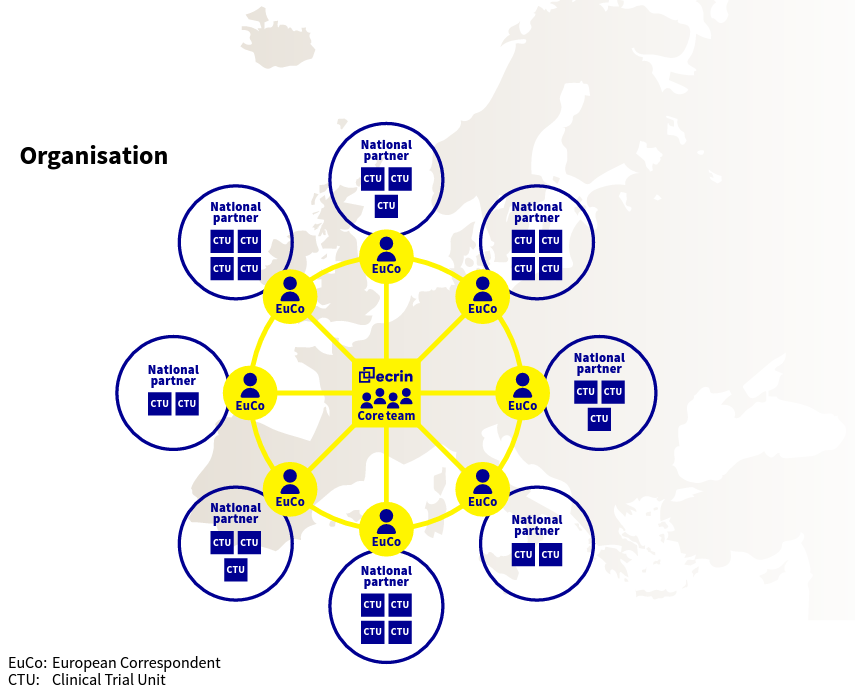 As the local partner of ECRIN, HECRIN works as a link between ECRIN core team and Hungarian parties involved in investigator-initiated clinical trials: HECRIN can connect investigators or local institutions to the international network and its supporting activities. HECRIN also develops and organizes a national network of Clinical Trial Units, academic institutions and clinical research service providers in Hungary with a view to strenghten the infrastructure for investigator-initiated trials in Hungary. Through the wide knowledge, deep experience and complementary competencies of HECRIN members they can contribute to the efficient implementation of academic clinical studies in Hungary.
As the local partner of ECRIN, HECRIN works as a link between ECRIN core team and Hungarian parties involved in investigator-initiated clinical trials: HECRIN can connect investigators or local institutions to the international network and its supporting activities. HECRIN also develops and organizes a national network of Clinical Trial Units, academic institutions and clinical research service providers in Hungary with a view to strenghten the infrastructure for investigator-initiated trials in Hungary. Through the wide knowledge, deep experience and complementary competencies of HECRIN members they can contribute to the efficient implementation of academic clinical studies in Hungary.
Contact:
Annamária NÉMETH, ECRIN European correspondent
+36 70 439 9272